Now and then it helps to have another look at the basics of one’s craft. This sort of exercise works to refresh our way of thinking about things. Lubrication is no different. No matter how much you know about friction, wear, oils and their classification, it’s worth reading again some good textbook passages about what we’re trying to achieve with lubricants.
Recently I bought a copy of Developments in Lubricant Technology by Dr S.P Srivastava, a former executive director of the R&D center of the Indian Oil Corporation. Dr Srivastava holds thirty patents and is the author or co-author of 200 research papers. What he has to say about lubrication, friction, and wear is clear, precise, and refreshing.
“The purpose of a lubricant is to reduce friction and wear between the two contacting surfaces….under the operating conditions of temperature, pressure, load, speed, stress, and time duration.”
Friction is the property of a material. Wear is the consequence of load and the relative movement of the two contacting surfaces.
Higher friction – resistance to motion – consumes more energy. Frictional resistance depends on the load applied to a surface. In other words, the better the understanding of the situation (metal surface material, load, speed, etc) the better a lubricant can be designed to reduce the load and thus reduce the friction.
All surfaces are not molecularly smooth. They have peaks or asperities, and valleys. It’s the asperities that require attention in understanding lubrication.
Metal surfaces react with air and form oxides. Sulfur, chlorine, and nitrogen becomes sulfides, chlorides, and nitrides. So the metal surfaces are complicated things. Layers or films develop which influence lubrication performance.
Have a look at Figure 1 below:
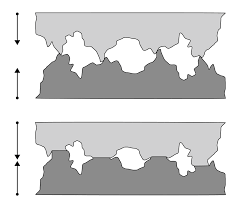
This image clearly illustrates the asperities and valleys of two metal surfaces in contact with each other. As the load increases, contact becomes broader. The contact area increases as the asperities are compressed.
Boundary friction is important here. With a high load between surfaces, a lubricant film may not be able to carry the load. Extreme pressure (EP) additives containing sulfur or phosphorous generate a fresh layer of surface capable of carrying the load. Likewise graphite or molybdenum disulfide can form a protective layer. So higher loads can be carried only when the contacting surfaces are separated by another molecular layer of a substance that can influence the friction and wear characteristics.
Adhesion wear occurs where the asperities of two contacting surfaces are in contact with each other. Such adhesion wear is reduced by a molecular layer formed by boundary additives. This gives rise to hydrodynamic or fluid lubrication: the fluid film must be three times higher than the average asperity height. When this is achieved it’s the viscosity of the lubricant that determines the friction.
“ Certain animal and vegetable oils, esters and compounds have better oiliness than mineral oils due to their polar nature. These polar molecules are asymmetrical and have a strong affinity for a metal surface, forming adsorbed layers at the rubbing metal surfaces. These layers can prevent a direct metal-to-metal contact in the bearing at moderate loads.”
All this is relevant to an understanding of SOD-1 Plus and its chemical and physical effects as a lubricant additive. Diagram 2 below illustrates how the micro-particles of SOD-1 Plus work to form that extra layer of protection.
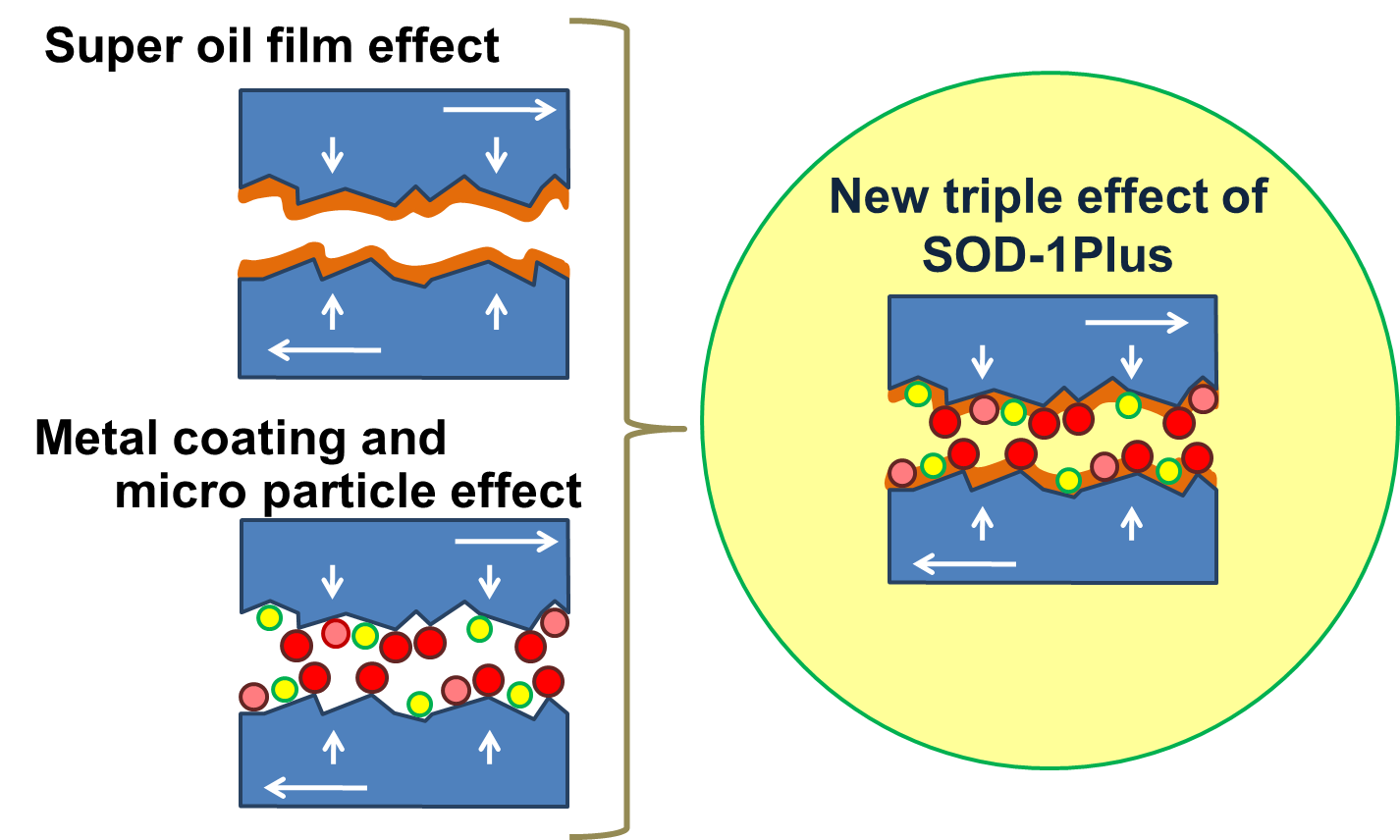
The yellow dots in the diagram represent the adsorption layer that SOD-1 Plus creates. This is the phosphorous and other chemicals sticking to the metal surfaces to add a layer to what the base oil already supplies.
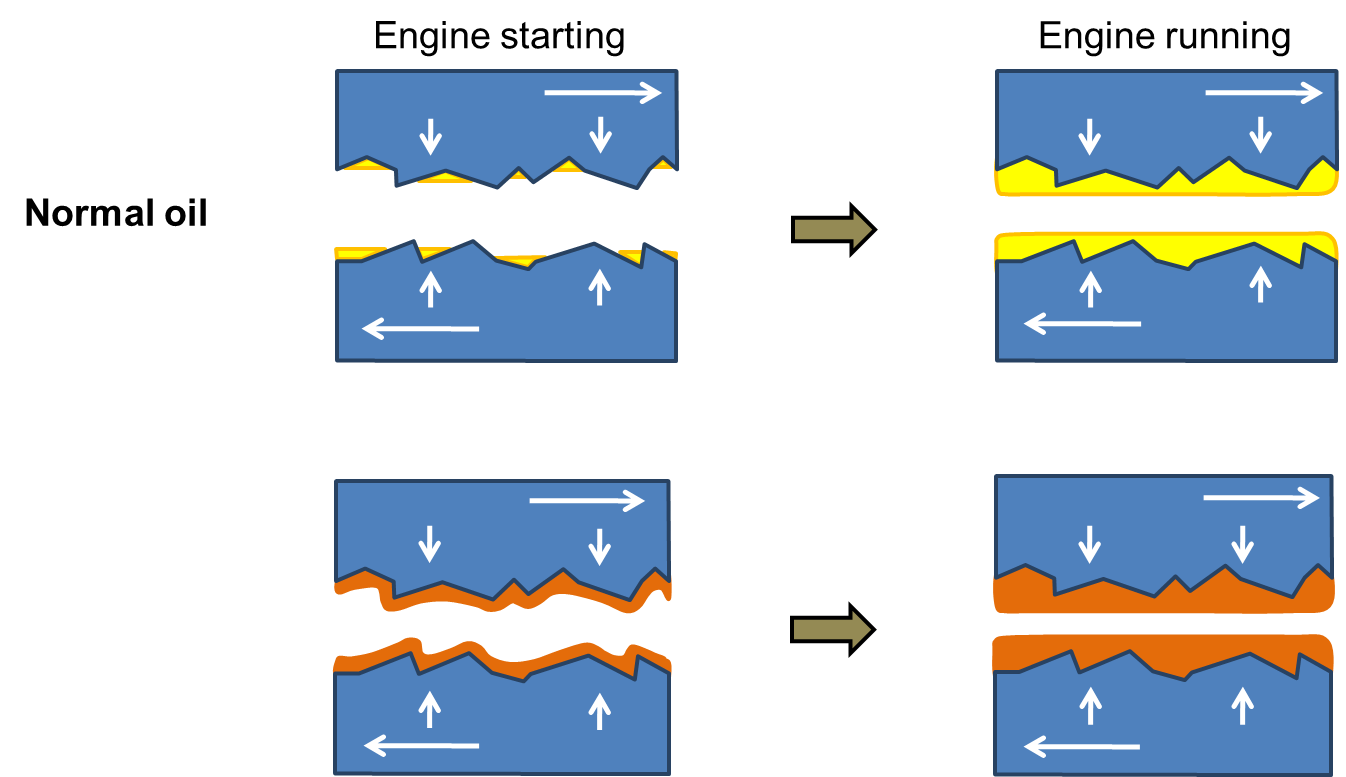
Diagram 3 below shows how this works in practice. When an engine is cold the asperities of the metal surfaces are bare of protection and you get a dry start: needlessly high friction and wear that damages the surfaces. The lower part of the diagram shows the effect of SOD-1 Plus in the orange-coloured layer that remains on the surfaces even when the engine is cold. That’s because of the adsorption, the chemical effect that Dr Srivastava described in the quote above, “…These polar molecules are asymmetrical and have a strong affinity for a metal surface, forming adsorbed layers at the rubbing metal surfaces….”