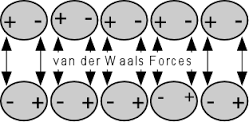
What do geckos, spiders, and SOD-1 Plus have in common? Answer – Van Der Waals Force, a theory of physical chemistry that lies behind adsorption.
We know that SOD-1 Plus works to provide an extra layer of oil film on metal surfaces because of adsorption: chemical polarity causes SOD-1 Plus to stick to metal more strongly than does base lubricant oil. There is opposite polarity between the molecules in SOD-1 Plus and the metal, causing them to attract.
So what is Van Der Waal’s Force? Encyclopedia Brittanica says:
“Van der Waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all organic liquids and solids. The forces are named for the Dutch physicist Johannes Diderik van der Waals, who in 1873 first postulated these intermolecular forces in developing a theory to account for the properties of real gases. Solids that are held together by van der Waals forces characteristically have lower melting points and are softer than those held together by the stronger ionic, covalent, and metallic bonds.
“ Because of fixed distortion in the distribution of electric charge in the very structure of some molecules, one side of a molecule is always somewhat positive and the opposite side somewhat negative. The tendency of such permanent dipoles to align with each other results in a net attractive force. “
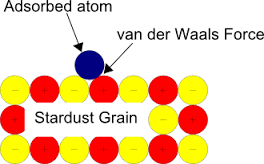
Professor Watanabe says simply, “ It’s a matter of static electricity.” The Wikipedia entry for Van Der Waals Force also carries an interesting note about geckos and spiders:
“ The ability of geckos – which can hang on a glass surface using only one toe – to climb on sheer surfaces has been attributed to the van der Waals forces between these surfaces and the spatulae, or microscopic projections, which cover the hair-like setae found on their footpads…… Some spiders have similar setae on their scopulae or scopula pads, enabling them to climb or hang upside-down from extremely smooth surfaces such as glass or porcelain.”